26. Metallic elements are more likely to lose electrons and form cations.
27. This indicates that they are hardly chemically reactive since their electrons are not attracted to any other element.
28. Anion or Cation:
a. Na: cation
b. Ca: cation
c. F: anion
d. Cu: cation
e. O: anion
f. Li: cation
g. Sn: cation
h. I: anion
29.Oxygen with mass number 16 and oxygen with mass number 18 is the more chemically similar pair. This is because only their masses are different, but they still have to same number of protons and electrons, and have the same charge.
30. The diameter of calcium should be around 205 pm.
31.Physical an chemical changes:
a. If a substance changes color then it could have combined with another substance chemically, or its temperature changed rapidly, causing the color to change temporarily.
b. A change in temperature could have happened because of freezing or boiling, or it could have been the result of combustion.
c. Gases formed from a chemical change could have come from a mixture with another substance, or it could be a physical change resulting from boiling and evaporation.
32. Elements:
a. Bromine (Br)
b. Silicon (Si)
33.The fish-kill river data and the periodic table are similar because they were arranged periodically, or in intervals.
34. For one example, argon and potassium would have been switched because argon’s weight (39.95) is slightly larger than potassium’s (39.10). Another example is cobalt and nickel, because Co’s weight id 58.93 while Ni’s is 58.69.
1. In Earth’s atmosphere supplies of nitrogen and oxygen are found in 78% and 21% compositions respectively. In the hydrosphere water and many dissolved minerals are contained. Lastly, in the lithosphere, which is the solid part of the Earth, provides a variety of chemical resources such as petroleum and metal-bearing ores.
2. Layers of the Earth:
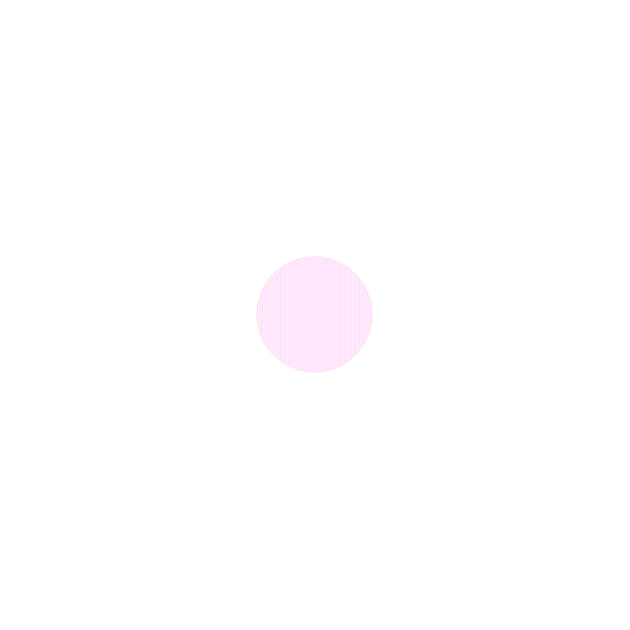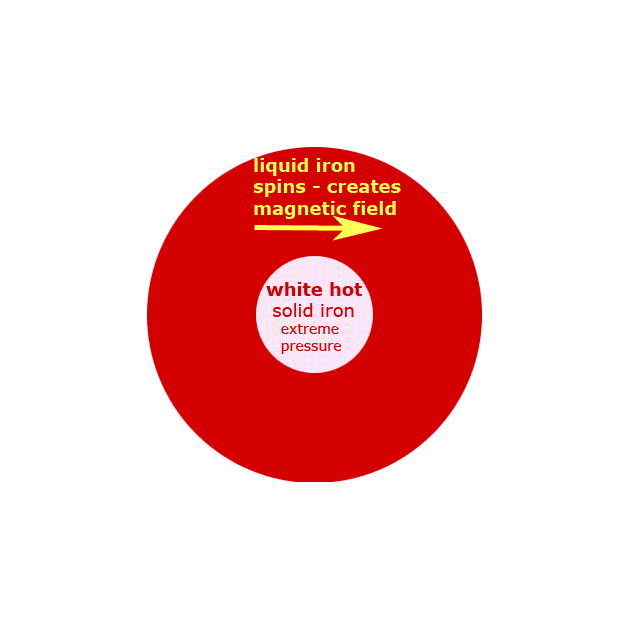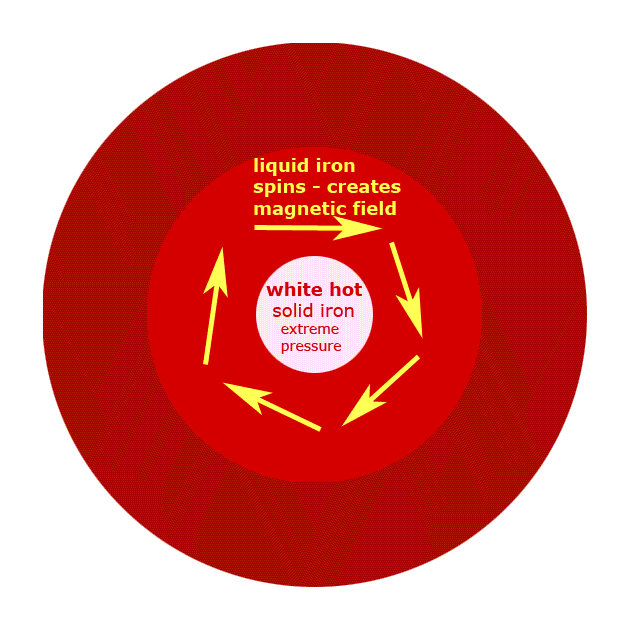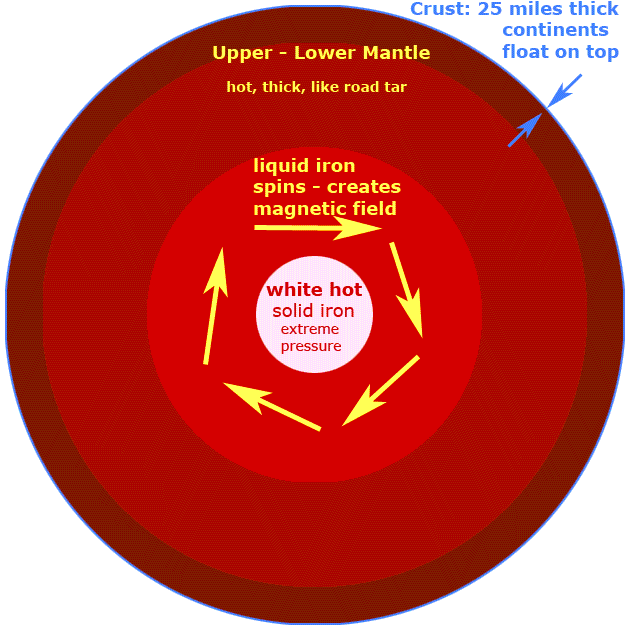
a. There are three major parts in the lithosphere, the crust, mantle and core. The crust is the outermost and thinnest layer of the earth where some silicates, coal, oil, natural gas, carbonates, oxides and sulfides are found. The mantle, the middle layer, contains silicates of magnesium and iron. Finally, the core is the innermost layer of Earth. It has very high temperatures and has iron and nitrogen.
b. The crust serves as the main storehouse of chemical resources used in manufacturing consumer products.
3. National productivity:
a. Mexico produces the most silver (0.1 metric tons more than Peru).
b. Japan produces the most copper.
c. China produces the most tin.
4. China produces the largest masses of aluminum, copper, iron ore, lead, nickel, silver, tin, and zinc.
5. Minerals are naturally occurring solid compounds containing the element or group of elements of interest. On the other hand, an ore is a naturally occurring rock or mineral that can be mined and from which it is profitable to extract a metal or other material.
6. The factors determining the feasibility of mining a particular ore are:
· The quantity of useful ore found at the site
· The percent of metal in the ore
· The type of mining and processing needed to extract the metal from its ore
· The distance between the mine and metal-refining facilities and markets
· The metal’s supply-versus-demand status
· The environmental impact of the mining and metal processing
7. The demand for gold might have increased so even if there is not a large percent of gold in the ores, it is economically possible to mine those ores.
8. A “useful ore” is one that contains a high percentage of the mineral being mined, as opposed to an ore that contains 4% of the mineral.

No comments:
Post a Comment